Testimonial: Car Accident
Ken shares his testimonial as a Shunnarah client after a car accident.
The Durom Cup hip replacement was temporarily pulled off the market when numerous reports of complications began to surface. This forced many injured patients to file a Zimmer hip replacement lawsuit in the hopes of recovering financial compensation.
Case evaluations are 100% free and available 24/7
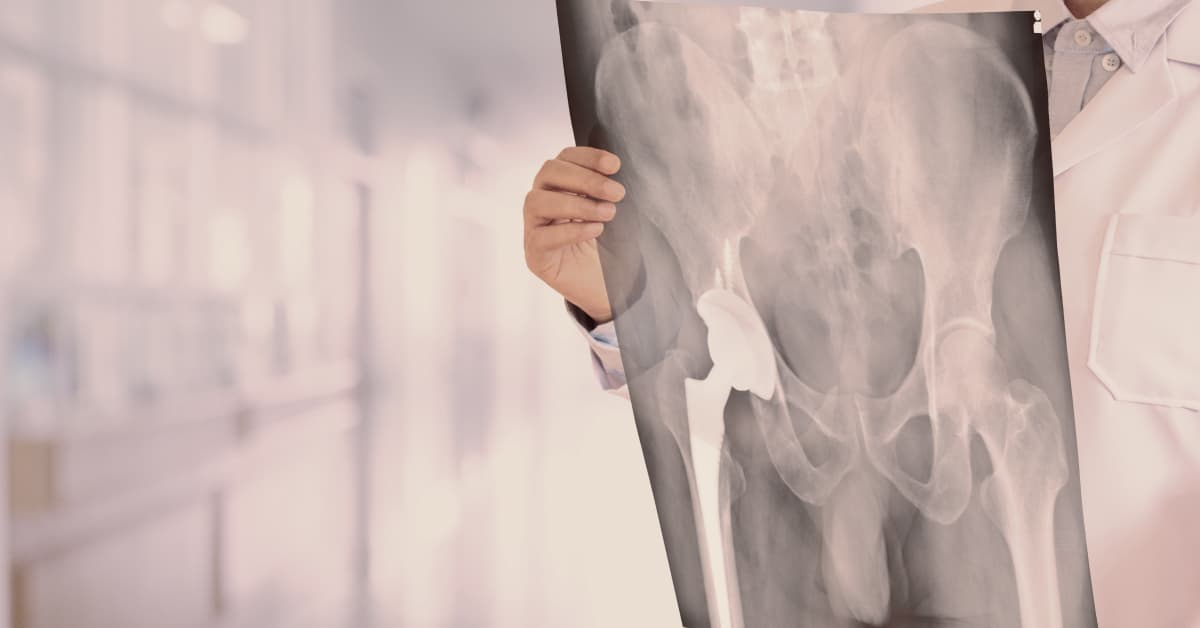
Zimmer Holdings Inc. is a global manufacturer of metal-on-metal hip implant products, including complete revision systems, femoral components, and acetabular components. The Durom Cup, one of many Zimmer products, was implanted in more than 12,000 patients from 2006 to 2008 alone.
Zimmer Holdings Inc. manufactures acetabular components that line the acetabular cup and femoral components that fit in the femur and create a new femoral head. Femoral components include the M/L Taper with Kinectiv Technology and the Versys Epoch FullCoat Hip, among others. Acetabular components include the Trilogy Acetabular Hip System and the Durom Cup. Zimmer also has complete hip revision systems like the Wagner SL Revision Hip.
If you have experienced adverse effects from your Zimmer Metal Hip Replacement, Shunnarah can help you with the following:
Many of Zimmer’s products are metal components and its revision systems are metal-on-metal (MoM), which means both the femoral head and acetabular lining are made of metal parts. Metal-on-metal hip components became popular in recent years as an alternative to traditional hip replacement systems that included ceramic components.
The hip joint consists of a femoral head, which rests atop of the femur. The joint fits inside of the acetabulum, which is a concave area in the pelvis. When a patient undergoes hip revision surgery, the acetabulum may be lined with a cup, which is referred to as an acetabular component. The patient may also have the femoral head replaced and a rod inserted into the femur to hold the new joint in place.
Metal-on-metal hip replacement systems were marketed primarily to younger consumers as a longer-lasting alternative that could provide a broader range of motion and more flexibility than traditional replacement joints. As many as 10 percent of all hip replacement procedures between 2006 and 2009 used MoM products and the majority of these procedures were performed on individuals aged 50 or younger. In fact, for patients within this age group, nearly half of all surgical hip replacement or revision procedures used metal-on-metal components.
Several of the metal-on-metal systems, including those produced and sold by Zimmer Holdings Inc., came to the market after going through the FDA’s 501(K) clearance process. This is a simpler, expedited approval process that permits the sale of a product with limited or no dedicated human testing. This is permitted because the product is considered very similar to existing medical devices on the market. The absence of testing, however, may have resulted in dangerous products being released onto the market.
In July 2008, Zimmer suspended sales of the Durom Cup because of a high rate of reported failures . Though not technically a hip recall, the company subsequently determined that surgeons were to blame for any problems and that the Durom Cup was not defective. After updating the surgical instructions, the product was re-released.
However, many medical experts believe this product and other Zimmer hip components are unsafe. Larry Dorr, the director of the Institute for Arthritis Research and Education, identified a high failure rate among certain Zimmer products, including the Durom Cup. Instead of lasting 15 years as promised, the devices were failing quickly, making revision surgery necessary. Dorr alerted the FDA and Zimmer to the failure rate, but the company continues to market and sell hip replacement products despite evidence of serious Zimmer hip complications.
Patients who are harmed by medical devices like Zimmer hip implants have legal rights. Zimmer Durom Cup lawsuits have been consolidated in multidistrict litigation (MDL) in the U.S. District Court of New Jersey. MDL coordinates similar civil cases before a single judge for pretrial proceedings. That judge can then issue rulings that apply to all pending claims, thus allowing cases to be handled more expediently.
MDLs are appropriate when plaintiffs have been harmed by a similar damaging product but have experienced different types of health problems as a result. For some plaintiffs who’ve brought a Zimmer hip lawsuit, the problem is a failure of the Durom Cup to adhere to the bone and remain in place, resulting in unexpected hip joint movement. For others, metallosis may occur. Metallosis is a type of metal poisoning that happens when the components rub against each other, releasing tiny fragments of chromium and cobalt, which can contaminate the body.
Other symptoms may include:
If you’ve experienced these symptoms and need an attorney, contact Shunnarah now.
Alexander Shunnarah Trial Attorneys handles cases in all 50 states. Find a location or attorney to assist you with your case.
Explore our FAQ to get the answers to some of our most frequently asked questions about Zimmer hip replacements.
What are common complications associated with Zimmer Hip Replacements?
Zimmer Hip Replacements, particularly certain models like the Durom Cup, have been associated with a range of complications that can lead to significant discomfort and mobility issues. Common problems include loosening of the implant, metallosis (metal poisoning from metal-on-metal components), premature failure of the device necessitating revision surgery, and severe pain around the hip area.
How do I know if my Zimmer Hip Replacement is failing?
Symptoms that may indicate a failing Zimmer Hip Replacement include persistent pain in the hip area, swelling, difficulty walking, and sometimes a popping or grinding noise from the hip joint. If you experience these symptoms, it’s essential to consult with your orthopedic surgeon who can perform diagnostic tests such as X-rays or MRI scans to assess the condition of your hip implant.
Am I eligible to file a lawsuit if I’ve had complications with my Zimmer Hip Replacement?
Eligibility to file a lawsuit generally depends on factors such as the specific model of your hip replacement, the nature and severity of your complications, and how these issues have affected your quality of life. If your complications can be directly linked to a defect in the Zimmer Hip Replacement, you may have grounds for a lawsuit. A consultation with a personal injury lawyer specializing in defective medical devices can provide clarity on your eligibility.
Available 24/7 for immediate case evaluations post-accident or injury.
Connect with a dedicated attorney who will aggressively pursue your compensation through trial if needed.
Receive top-tier legal guidance and representation. We don’t give up until you receive the compensation you deserve.